- syed.nasar@daneenbio.com
- +91-9321378847
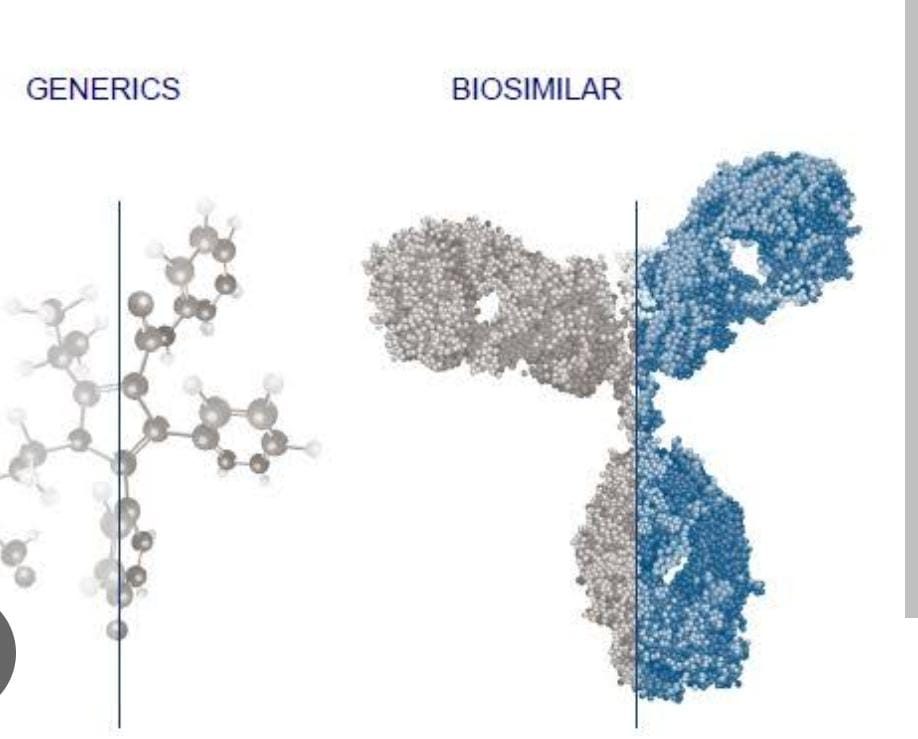
BIOSIMILARS
Dawn of A new Era of choice in Medical therapy .
- There is an URGENT need for a Comprehensive support and education to navigate the evolving biosimilars landscape and build broader understanding /consensus of the role biosimilars play in facilitating high quality, lower cost care in life threatening diseases .
- Pharmacists are among the most trusted and relied-upon healthcare providers. With the introduction of interchangeable biosimilars, the role of the pharmacist and Pharmacies will become even more critical as patients reach out with questions about how biosimilars may impact their treatment options or costs. We at DANEEN BIOTHERAPEUTICS have the ACUMEN to help you navigate this emerging area of therapy.
- In terms of the global market, USFDA has already revised its regulations to eliminate the outdated biologics requirement which is allowing innovators and manufacturers to explore new technologies to boost the production of biologics. The continuous updating of regulatory requirements by USFDA for biologics approval is expected to increase regulatory flexibility in the global pharma industry.
- Moreover, patent expiration of blockbuster biologics and governments’ thought to reduce the healthcare cost by introducing biosimilars (highly similar copies of biologics) in the market is further fuelling the Go-to-global-market excellence amongst the pharma and biotech industry.
- Biosimilars hold promise to improve patient’s accessibility for many malignant and nonmalignant ailments by reducing the treatment cost. Since the use of the first biosimilar, the development and uses of “biosimilars or similar biologics” have witnessed substantial growth. Every year, regulatory agencies are granting approval of various similar biologics for the treatment of many cancerous and noncancerous diseases. India has firmly established itself as a global player as a maker of similar biologics. It is also a huge market for the similar biologics because of its burgeoning population.
Example of some of the biosimilars approved in India
| Products Name | Active Drug | Indications |
|---|---|---|
| Glarituse | Insulin glargine | Diabetes mellitus |
| Grafeel | Filgrastim | Neutropenia |
| Epofer | Epoetin alfa | Anemia |
| Adfar | Adalimumab | RA, Crohn’s disease |
| Erbitux | Cetuximab | Colorectal carcinoma |
| Krabeva | Bevacizumab | Colorectal cancer |
| Herceptin | Trastuzumab | Breast cancer |
| Intacept | Etanercept | RA |
| Abcixirel | Abciximab | Autoimmune disease |
| Relibeta | Interferon beta-la | Multiple sclerosis |
| Shankinase | Streptokinase | Anemia, autologous blood transfusion,chronic kidney failure, HIV |
| Rajumab | Ranibizumab | Arterial occlusions, deep-veinthrombosis, pulmonary embolism |
| Terifrac | Teriparatide (parath}7oid hormone) | Wet macular dege.neration, macular edem; degenerative myopia |
| Poronen. opausal women with osteoporosis who are at high risk for fracture |
